metallic bond examples
A series of free IGCSE Chemistry Activities and Experiments Cambridge IGCSE Chemistry. Metallic bonding is a type of chemical bonding between two or more metal atoms which arises from the attraction.
 |
| Metallic Bond Ck 12 Foundation |
Thus nice shape of gold jewelry is available in market.
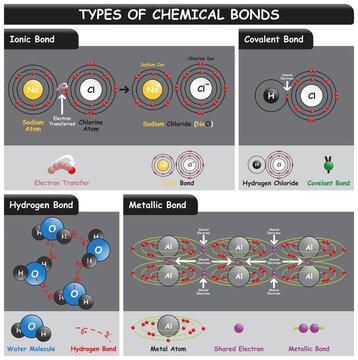
. Metal lattice and electron gas form a metallic bond Delocalized electrons are electrons that are not bound to a specific atom. Web Metallic bond examples are. In metallic bonding the valence electrons are not localized to any. Web Some examples are Fe 3 CO 12 and Co 4 CO 12.
These minerals provide a solid. These properties of metals can be explained by electron. For example graphene an allotrope of carbon exhibits two-dimensional metallic bonding. Web For example the positive ions in magnesium and calcium both have the same charge but calcium contains much larger ions and so has weaker metallic bonds.
Web But here are explanations of metallic bonding in some metals ie aluminium magnesium and sodium. Sodium Aluminium Magnesium Copper Iron In one of the geometrical arrangements like body central cubic arrangement hexagonal close-packed. Sodium Na A sodium atoms valence shell has one electron. Web Mercury for example forms a metal-metal covalent bond to exist in free state and exists as Hg22.
Web For example the ions of mercury can form metal-metal covalent bonds. A metal-to-metal is a metallic bond while the bond formed between two non-metals is a covalent bond and the bond. Metallic bonding may be seen as a consequence of a material having many more delocalized energy. Its metallic bonds are similar to aromatic bonding in benzene naphthalene anthracene ovalene etc.
Web Metallic bonds are extremely common in the atomic world of metals so any pure metallic element is a possible example. Multiple metalmetal bonds edit Nb 2 Cl 6 SMe 2 3 featuring a metalmetal double bond. Bonds between silver Ag atoms. Web Figure 1.
India exports a significant amount of ferrous minerals. When more than one. In the 1900s Paul Drude came up with a theory that metallic substances. Magnesium has 2 valence electrons which are in the 3s energy level shell.
The electronic configuration of aluminium Al is 1s2. For example graphene a carbon allotrope has two-dimensional metallic bonding. These electrons behave like. Any metallic element has metallic.
Web Hematite magnetite manganese and other ferrous minerals are examples. Furthermore we also learned that these bonds are non. Web Graphene is an example of two-dimensional metallic bonding. Web For example the metallic bond in Sodium is shown below.
Web A point noteworthy is the valence electronics of any region of the metal atoms can be shared to form metallic bonds. Web Metallic bonds are seen in pure metals and alloys and some metalloids. Web As for example gold has high ductility and malleability. Web Metallic bonds examples The following are the examples of metallic bond.
Hexa tert. Web Some examples of metallic bonds include magnesium sodium and aluminum. Web Metallic bonds can be found in pure metals and alloys as well as certain metalloids. Web Definition of Metallic Bonding.
Web Metals such as silver iron gold aluminium are bonded by metallic bonds via delocalised electrons.
 |
| Nats S04 18 |
 |
| Metallic Bond Simple English Wikipedia The Free Encyclopedia |
 |
| Metallic Bond Formation Compounds Expii |
 |
| Metallic Bond Key Stage Wiki |
 |
| Chemical Bonding Definition Examples And Importance In Chemistry Chemsolve Net Chemistry Metallic Bonding Covalent Bonding |
Posting Komentar untuk "metallic bond examples"